How To Build A Camera Case
How to build a low-cost underwater camera housing for aquatic research
Publication: FACETS
16 February 2017
Abstruse
Remote cameras are an increasingly important tool in field-based biological research. Terrestrial researchers can purchase inexpensive off-the-shelf cameras, simply aquatic researchers face challenges in adopting similar systems for underwater scientific discipline. Although technology allows researchers to deploy cameras in any aquatic environment, high procurement costs are often a barrier, especially for studies that require the collection of lengthy videos. In this annotation, we provide a detailed guide explaining how to assemble an underwater camera system for less than $425 USD. We focus especially on the construction of the underwater housing, which is typically the well-nigh expensive component of an underwater camera system. Equally described, this system can record 13 h full high-definition videos in depths up to 100 m. It tin can be constructed and assembled with express technical background using tools available in most workshops. The guide includes a general overview of the organisation, a full list of components, detailed instructions on amalgam the camera housing, and suggestions on how to mount and use the camera in fieldwork. Our goal for this annotation is to promote the wider use of remote underwater cameras in aquatic research by making them accessible to those with limited fiscal ways.
Introduction
Aquatic science relies on technologies that provide information most the underwater surround. Underwater cameras are a widely used tool to perform direct observations on the behaviour of organisms in their native habitat and tin can greatly supplement the data derived from other ways. As an instance, cameras can be used to study the behaviours of organisms that interact with static (e.1000., Renchen et al. 2012; Bacheler et al. 2013; Favaro et al. 2014) and mobile fishing gear (due east.g., Nguyen et al. 2014; Underwood et al. 2015) in situ.
The merits of applying camera systems in biological studies take been amply demonstrated in the terrestrial realm, where a mature body of testify has described their application to studying ecosystems (O'Connell et al. 2011). Many of these systems are inexpensive and readily available equally off-the-shelf products, which has assisted in the proliferation of this study technique. In dissimilarity, underwater camera systems can be substantially more expensive, due primarily to the challenges associated with operation in an aquatic surroundings.
The utility of underwater cameras for aquatic scientific discipline depends in office on the specifications and capabilities of the camera gear (Favaro et al. 2012; Underwood et al. 2012; Struthers et al. 2015). For cameras that are self-independent (i.e., practice not need to be connected to a gunkhole or other fixed power source at the surface), there are essentially two categories: expensive systems that are highly capable; and inexpensive systems that are limited in bombardment life, safe operating depth, storage capacity, and low-lite sensitivity (due east.g., Struthers et al. 2015). For researchers that need to tape video for longer periods of time (e.thou., studying deployed fishing gear), systems specific to the project oftentimes take to be designed and built (e.g., Jury et al. 2001; Bacheler et al. 2013). Trial-and-error associated with the pattern and structure process tin add to the price of underwater projects and prevent them from being completed in a timely manner. This can result in researchers fugitive the use of underwater cameras, or selecting off-the-shelf cameras that can merely record for short durations due to limitations in battery life.
In this note, nosotros depict a camera system that nosotros constructed from components available at full general hardware and consumer electronics stores. At that place are four components to this organization: the camera itself, the external battery pack, the housing, and the mounting frame connected to the fishing gear (or other structure) under examination. Nosotros devote most of this note to describing the construction of the underwater camera housing, which is often the most expensive component to procure when assembling a camera system for aquatic research. The underwater housing described in this note was adjusted from a blueprint shared on a hobby website (Anonymous 2006). The total cost of components for ane housing was $145 USD (Supplementary Material one).
With proper intendance taken in construction, the housing is capable of beingness safely used at depths up to 100 m. It is large enough to comprise a small camera such as a Sony Action Cam with an additional battery pack, and the configuration we draw in this note is capable of recording total high-definition videos at 1080p resolution for thirteen h (Sony Corporation 2014). This note provides sufficient particular for someone with limited technical experience to procure the materials and construct the underwater photographic camera housing. Our promise is that this note empowers researchers to construct inexpensive housings that facilitate the collection of long-elapsing video recordings in aquatic environments.
Materials and methods
Camera components and necessary tools
We selected a Sony HDR-AS20 Action Cam and attached an Anker Astro E4 13 000 mAh bombardment pack (any battery pack with at least a 10 000 mAh capacity and a USB connectedness should suffice) to increment battery life and recording duration. Using a 128 GB Micro SDXC memory card, we were able to record continuously for 13 h at 1080p resolution.
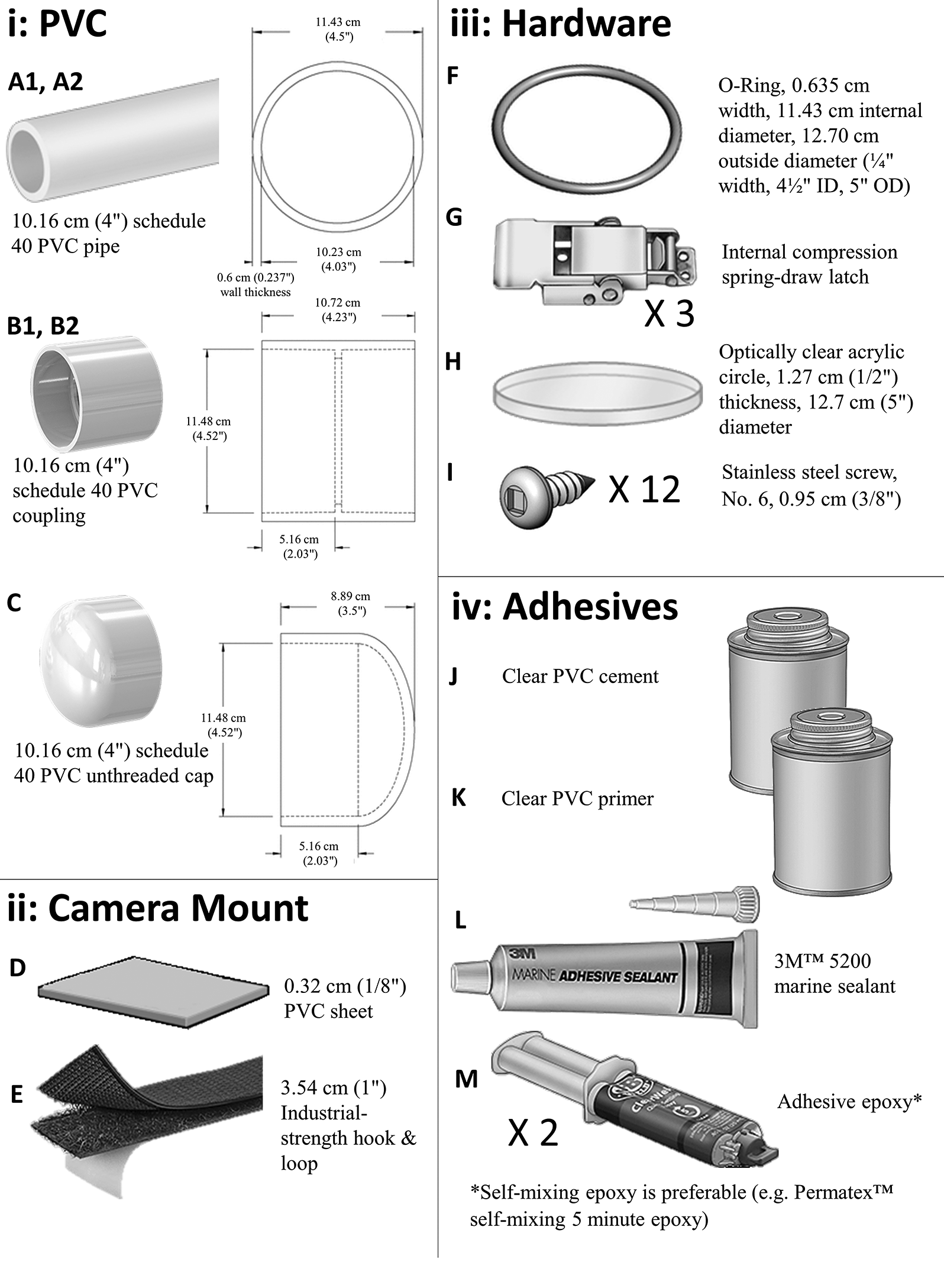
The tools required for this project comprise: pencil, mark, record measure, ruler, calipers, miter saw equipped with a plastic-cutting blade, ring saw, lathe, scissors, fine sandpaper, masking tape, iv 2 mm shims (nosotros propose using coins), cord, steel punch, drill press, 1.95 mm (v/64″) drill flake, and a No. 1 dark-green Robertson screwdriver. Figure 1 shows a completed housing, and Fig. two shows the raw materials and hardware needed for its structure. Finally, Supplementary Material ii demonstrates how to execute each step of the structure process.
Fig. 1.
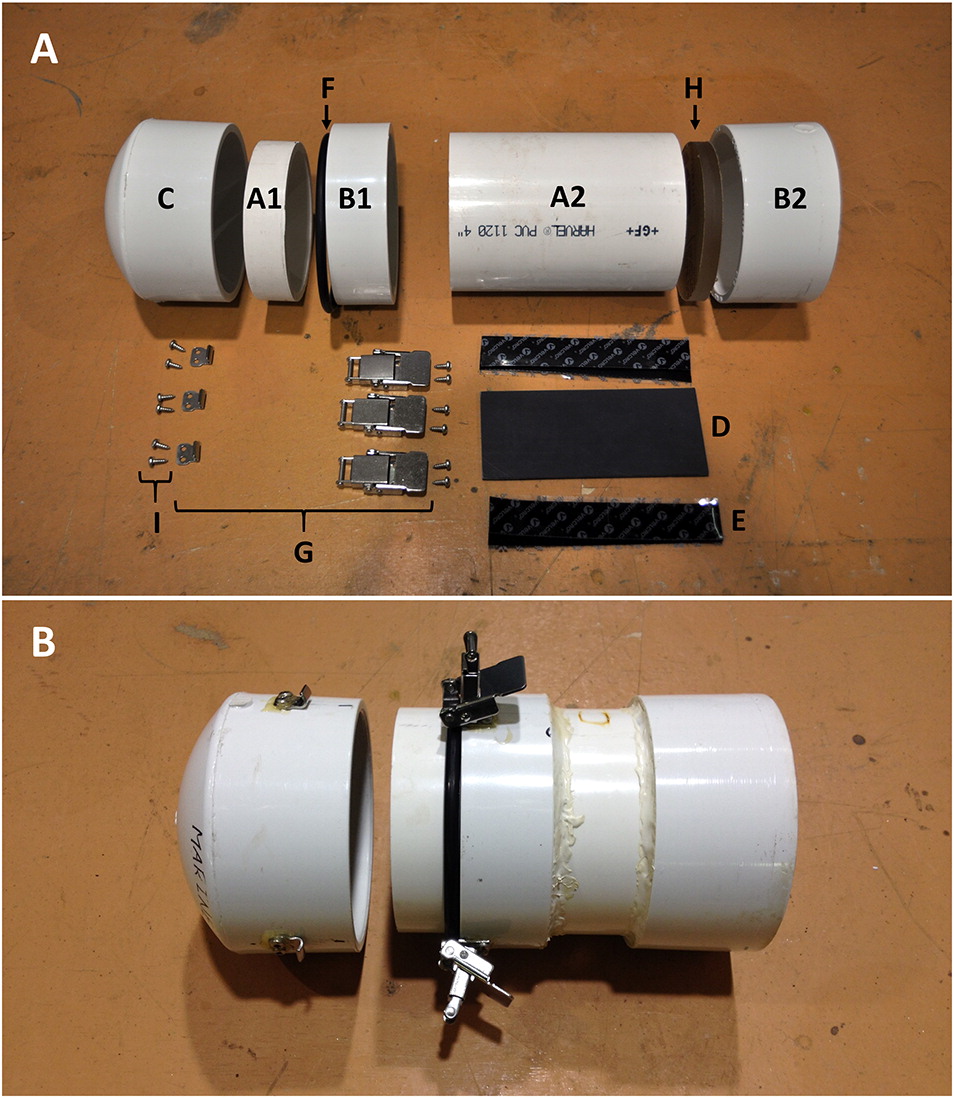
Fig. 2.
Structure of the underwater housing
To construct the underwater housing, detailed instructions are provided in Supplementary Material three with accompanying photos and text describing how to complete each step. We recommend using these instructions while reviewing the accompanying video (Supplementary Material two) to assist in the structure process. These instructions guide the researcher through preparation of all the necessary components and assembly of the completed housing (Fig. 1). We also include a description of each component and its cost at the time of writing (Supplementary Material 1).
The housing itself consists of two chief parts: the trunk of the housing and the end cap that latches firmly onto the body to class a watertight seal. The body contains the camera and external battery, and features a articulate, acrylic viewport on the forepart stop. The housing is constructed using 10.16 cm bore (four″) schedule 40 (0.half-dozen cm wall thickness) PVC piping components. We used schedule twoscore PVC components as they are inexpensive, widely available, and durable. The components required to construct the housing are widely available at most hardware stores, and the housing can exist constructed and assembled with express technical groundwork using tools bachelor in most workshops.
Construction of the housing does not take a large amount of fourth dimension and is mostly express by the time required for the epoxies and adhesives to fully cure. For example, the last step in structure of the housing requires the use of 3M™ 5200 marine adhesive sealant, which specifies a cure time of 5–vii d. We approximate that preparation of the component pieces and actual assembly time should accept approximately four–v h.
Utilise in the field, care, and maintenance
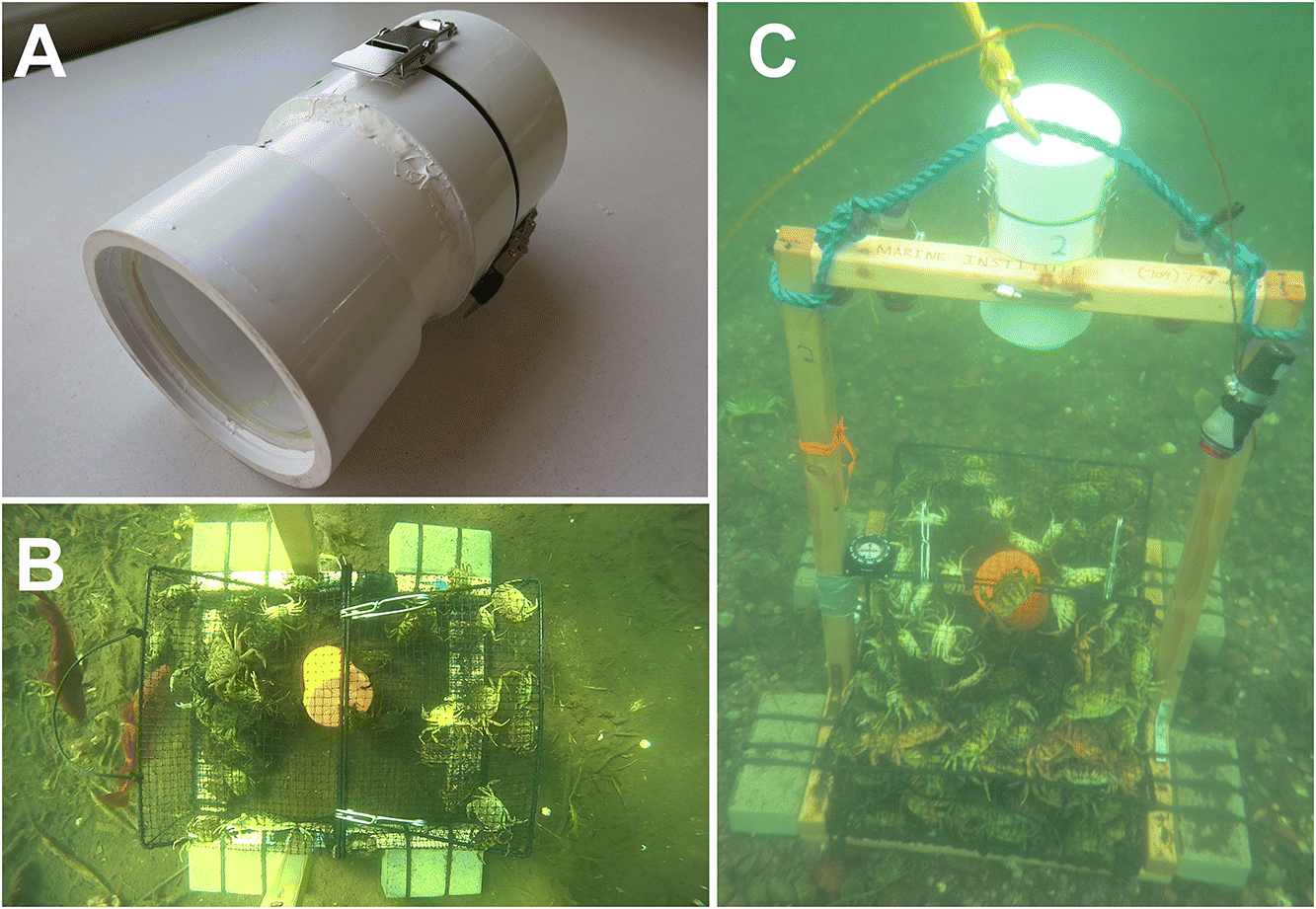
The underwater housing can be affixed to fishing gear or other structures. For our enquiry projection, we built wooden frames to mountain the camera systems to crab pots, similar to the designs of Jury et al. (2001) and Favaro et al. (2012) (Fig. three). We held the underwater housing firmly in place using a big 114–165 mm diameter gear clamp that allowed for easy mounting and removal of the camera housing. Depending on the application, researchers must consider whether to add external lighting. If external lighting is used, we recommend using lights capable of consistent illumination for the duration of video recording (to avert illumination problems such as those experienced by Favaro et al. 2012). We also recommend red lights, as they are less visible to many marine organisms than total-spectrum lights (Goldsmith and Fernandez 1968; Widder et al. 2005; Weiss et al. 2006). In our specific awarding, we used Light and Motion (Marina, California, USA) Gobe Plus flashlights equipped with red light attachments (the Gobe Focus Caput). We found information technology preferable to use lights with cherry LEDs, as opposed to white LEDs covered by a cherry filter, because the red LED uses less free energy to produce the aforementioned intensity of low-cal.
Fig. three.
The underwater housing requires petty maintenance. Although many housings require the utilise of O-band grease to facilitate the seal, we practise not recommend doing and then with our organization because nosotros found that it can increment the gamble of a leak. If the O-ring is kept clean and free from debris, compressional force alone is enough to maintain a water-tight seal. Be sure to inspect the O-ring regularly for signs of wear and damage. Additionally, if the housing is being used in a common salt h2o surroundings, it is important to rinse it thoroughly in fresh water after each use.
Hydrostatic pressure testing
The maximum condom operating depth for these housings depends on the care taken during the construction procedure. Therefore, we recommend that all housings exist tested for leaks either in a hydrostatic chamber if bachelor or in the field at-depth earlier installing a camera inside. We recommend testing empty camera housings at depths exceeding those intended for use in the study.
To measure the maximum rubber operating depth of this housing design, nosotros used a hydrostatic testing chamber. We tested two housings: one that was built to the exact specifications outlined in this notation (housing A); and another that was congenital the same manner, only with less sealant around the inside and outside of the viewport (housing B). We tested each housing separately in the hydrostatic chamber past steadily increasing the hydrostatic force per unit area to the point of failure (i.east., leakage or implosion). We applied water-finding paste effectually the viewport and O-band seal on the within of each camera housing to determine the entry point of leaks. Any contact with h2o would change the colour of the paste from grey to purple and would indicate the location of a leak. Deadening leaks within the chamber were indicated by a gradual drib in force per unit area within the chamber, whereas a catastrophic leak (east.k., implosion) would exist indicated by an instantaneous drop in force per unit area.
Results
Nosotros have used these underwater housings to conduct field-based enquiry in coastal waters of Newfoundland, Canada during the summer months of 2015 and 2016 to assess the efficiency of two types of fishing gear designed to catch European green crab (Carcinus maenas) and American lobster (Homarus americanus), respectively. Field inquiry was conducted under Experimental Licenses NL-3133-15 and NL-3271-xvi issued by Fisheries and Oceans Canada. The field research only involved invertebrates; therefore, it was classified equally a Category A study and was registered with Memorial Academy's Animal Intendance Committee. Nosotros built a total of seven camera systems that were deployed a total of 160 times, for a combined full of 2794 h underwater. Cameras were deployed in depths ranging from 2 to sixteen m, anywhere from 6 to 26 h duration. Across the entire written report, we experienced an incident where iii camera housings experienced minor leaks on the same day in 2015. We identified that the leakage was acquired past the use of O-band grease. Compression forced the greased O-ring out of place, which compromised the seal and caused the leak. Every bit a result, nosotros did not use O-ring grease during whatever other deployments and experienced no further bug.
Hydrostatic pressure level testing results
Housing A experienced a wearisome leak at 4760 kPa (equivalent to a depth of ∼470 grand), and housing B experienced a dull leak at 2000 kPa (equivalent to a depth of ∼200 m). The water-finding paste indicated that in both photographic camera housings the leaks occurred effectually the viewport. This large difference demonstrated the importance of taking great intendance to seal the lens thoroughly. This also suggested that increasing the thickness of the camera housing (e.yard., past using schedule 80 PVC) would non necessarily increase the depths at which this design could operate.
Give-and-take
Electronics change apace, but the demand to keep them dry out does not. It is always necessary to procure an underwater housing to record in situ video, and these housings are often among the most expensive parts of a camera system. It is our observation that although the video quality of off-the-shelf cameras has improved, and cameras have shrunk, they have not improved in their ability to record long-duration videos equally bombardment life and storage chapters are still limiting factors. It is notable that newer action cameras are capable of recording in 4K resolution, but these videos are more retention intensive than standard loftier definition. In improver, aquatic researchers are more express than their terrestrial counterparts in their ability to rely on movement sensors because of the frequent movement of currents, vegetation, and particulate matter which contribute to false-positive activations of the camera.
For our system, the full component costs at the fourth dimension of writing were $145 USD for each housing, $320 USD for the camera, memory card, and battery, and $xl USD for materials for the wooden frame used to mount the cameras to our detail traps. The lighting solution that we used price $650 USD. The most cost-effective fashion to produce this housing is to build more one at a fourth dimension because many materials (e.yard., PVC pipage, O-rings) are sold in pre-set up quantities or lengths, so this note will leave the researcher with an backlog of materials.
If researchers simply demand to record brusque shallow h2o videos in well-lit environments, and so an action camera with an off-the-shelf housing is sufficient (see Struthers et al. 2015 for review). Time-lapse cameras are cheap and widely available, merely they do non allow the user to tape long, continuous videos, which is a necessity for sure applications. In addition, most industrial fishing occurs in h2o likewise deep for nearly consumer photographic camera systems (Morato et al. 2006), so accessing these depths for the length of fourth dimension needed for fisheries inquiry is more challenging. We see this housing equally filling an important niche: an extremely low-toll solution for researchers that need to record long videos at shallow to moderate depths and that practice not accept access to the necessary funds to purchase specialty equipment. In addition, although construction feel is helpful (especially in the use of a lathe), we believe that a relatively depression level of pre-existing technical skill is needed to follow these instructions in comparison with other do-it-yourself camera options currently available (e.chiliad., Cazenave et al. 2014).
There are many uses for camera systems in underwater environments and their flexibility is only limited past the creativity of the researcher. To list a few applications, underwater cameras can be used to study fish behaviour (Hammar et al. 2013; Binder et al. 2014; Domenici et al. 2014), monitor interactions with line-fishing gear (Jury et al. 2001; Bacheler et al. 2013; Robbins et al. 2013), evaluate bycatch reduction devices (Favaro et al. 2013; Cairns et al. 2014; Lomeli and Wakefield, 2014), assess abundance and community structure (Clarke et al. 2012; Harasti et al. 2014), or map habitat and bathymetry (Schmidt and Rzhanov 2012). However, many studies involving action cameras are still limited past the elapsing and quality of video they are able to record. We are hopeful that this detailed note will get in easier for researchers to procure and use underwater cameras in their own research to facilitate long-elapsing video recordings in the underwater surround.
Acknowledgements
This projection was funded by a Marine Environmental Observation Prediction and Response Network (MEOPAR) grant to BF (grant EC1-BF-MUN), every bit well as by the Canadian Centre for Fisheries Innovation (H-2015-06) and the Government of Newfoundland and Labrador's Department of Fisheries and Aquaculture (NH-77863). NZ and JAB were supported by Ocean Industry Student Inquiry Awards from the Research and Development Corporation of Newfoundland (5404-1914-101 and 5404-1915-101, respectively). We give thanks Cynthia McKenzie, Kiley All-time, and Kyle Matheson for support in the field. We besides give thanks the Fish, Food, and Allied Workers Marriage, ACAP Humber Arm, Fisheries and Oceans Canada, Memorial University's Field Back up Services, and the Bonne Bay Marine Station. We thank Terry Bungay and Craig Hollett for contributing to hydrostatic testing of camera housings. Music for supplementary video was provided by www.bensound.com under the creative commons CC BY-ND iii.0 Unported license. Finally, we thank two anonymous reviewers and Paul Winger for their thorough and timely feedback, which greatly improved this manuscript.
References
Bacheler NM, Schobernd ZH, Berrane DJ, Schobernd CM, Mitchell WA, and Geraldi NR. 2013. When a trap is not a trap: converging entry and leave rates and their upshot on trap saturation of blackness sea bass (Centropristis striata). ICES Journal of Marine Scientific discipline, 70: 873–882.
Folder TR, Thompson HT, Muir AM, Riley SC, Marsden JE, Bronte CR, et al. 2014. New insight into the spawning behavior of lake trout, Salvelinus namaycush, from a recovering population in the Laurentian Cracking Lakes. Environmental Biology of Fishes, 98: 173–181.
Cairns NA, Stoot LJ, Blouin-Demers G, and Cooke SJ. 2014. Refinement of bycatch reduction devices to exclude freshwater turtles from commercial fishing nets. Endangered Species Enquiry, 22: 251–261.
Cazenave F, Kecy C, Risi Thousand, and Haddock Southward. 2014. SeeStar: a low-cost, modular and open-source camera system for subsea observations. In Oceans 2014, St. John'south, Newfoundland and Labrador, 14–nineteen September 2014. pp. i–7.
Clarke C, Lea J, and Ormond R. 2012. Comparative abundance of reef sharks in the Western Indian Bounding main. In Proceedings of the twelfth International Coral Reef Symposium, Cairns, Australia, 9–13 July 2012.
Domenici P, Wilson ADM, Kurvers RHJM, Marras S, Herbert-Read JE, Steffensen JF, et al. 2014. How sailfish use their bills to capture schooling prey. Proceedings of the Purple Society of London B: Biological Sciences, 281(1784): 20140444.
Favaro B, Duff SD, and Côté IM. 2013. A trap with a twist: evaluating a bycatch reduction device to prevent rockfish capture in crustacean traps. ICES Periodical of Marine Science, lxx: 114–122.
Favaro B, Duff SD, and Côté IM. 2014. Density-dependent catchability of spot prawns (Pandalus platyceros) observed using underwater video. Journal of Ocean Engineering, 9: 83–97.
Favaro B, Lichota C, Côté IM, and Duff SD. 2012. TrapCam: an inexpensive camera system for studying deep-water animals. Methods in Environmental Development, 3: 39–46.
Goldsmith Thursday, and Fernandez HR. 1968. Comparative studies of crustacean spectral sensitivity. Zeitschrift für vergleichende Physiologie, lx: 156–175.
Hammar 50, Andersson S, Eggertsen 50, Haglund J, Gullström 1000, Ehnberg J, et al. 2013. Hydrokinetic turbine furnishings on fish swimming behaviour. PLoS ONE, 8: ane–12.
Harasti D, Gallen C, Malcolm H, Tegart P, and Hughes B. 2014. Where are the little ones: Distribution and abundance of the threatened serranid Epinephelus daemelii (Günther, 1876) in intertidal habitats in New S Wales, Commonwealth of australia. Journal of Applied Ichthyology, 30: 1007–1015.
Jury SH, Howell H, O'Grady DF, and Watson WH. 2001. Lobster trap video: in situ video surveillance of the behaviour of Homarus americanus in and around traps. Marine & Freshwater Research, 52: 1125–1132.
Lomeli MJM, and Wakefield WW. 2014. Examining the potential utilize of bogus illumination to raise chinook salmon escapement out a bycatch reduction device in a Pacific hake midwater trawl. Bycatch Reduction Engineering Plan study, 61–66.
Morato T, Watson R, Pitcher TJ, and Pauly D. 2006. Line-fishing down the deep. Fish and Fisheries, vii: 24–34.
Nguyen TX, Winger PD, Legge Chiliad, Dawe EG, and Mullowney DR. 2014. Underwater observations of the behaviour of snow crab (Chionoecetes opilio) encountering a shrimp trawl off northeast Newfoundland. Fisheries Research, 156: ix–xiii.
O'Connell AF, Nicols JD, and Karanth KU. 2011. Photographic camera traps in animal ecology: methods and analysis. Springer, New York Urban center, New York. 271 pp.
Renchen GF, Pittman SJ, and Brandt ME. 2012. Investigating the behavioural responses of trapped fishes using underwater video surveillance. Journal of Fish Biology, 81: 1161–1625.
Robbins WD, Peddemors VM, Broadhurst MK, and Grey CA. 2013. Hooked on angling? Recreational angling interactions with the critically endangered grayness nurse shark Carcharias taurus in eastern Australia. Endangered Species Research, 21: 161–170.
Schmidt VE, and Rzhanov Y. 2012. Measurement of micro-bathymetry with a GOPRO underwater stereo photographic camera pair. In Ocean 2012 MTS/IEEE Harnessing Power Ocean, 14–19 Oct 2012, Virginia, USA.
Struthers DP, Danylchuk AJ, Wilson ADM, and Steven JC. 2015. Action cameras: bringing aquatic and fisheries research into view. Fisheries xl, 502–512.
Underwood MJ, Winger PD, Fernö A, and Engås A. 2015. Behavior-dependent selectivity of yellowtail flounder (Limanda ferruginea) in the mouth of a commercial lesser trawl. Fishery Bulletin, 113(4): 430–441.
Underwood MJ, Winger PD, and Legge Yard. 2012. Development and evaluation of a new high definition self-contained underwater camera organization to find fish and fishing gears in situ. Periodical of Ocean Technology, vii(one): 60–seventy.
Weiss HM, Lozano-Alvarez E, Briones-Fourzan P, and Negrete-Soto F. 2006. Using red calorie-free with fixed-site video cameras to study the behavior of the spiny lobster, Panulirus argus, and associated animals at night and inside their shelters. Marine Engineering science Society Journal, xl: 86–95.
Widder EA, Robison BH, Reisenbichler KR, and Haddock SHD. 2005. Using cherry-red light for in situ observations of deep-sea fishes. Deep Sea Research Part I: Oceanographic Research Papers, 52: 2077–2085.
Supplementary Material
Detailed list of hardware for housing, product numbers for an online hardware retailer, and price at fourth dimension of writing.
Fully annotated structure guide, including photos demonstrating each step.
Information & Authors
Information
Published In

FACETS
Book 2 • Number i • Jan 2017
Editor: Sophia Johannessen
History
Received: 12 September 2016
Accustomed: 13 December 2016
Published online: 16 Feb 2017
Copyright
© 2017 Bergshoeff et al. This work is licensed under a Creative Eatables Attribution iv.0 International License (CC BY four.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author(south) and source are credited.
Information Availability Statement
All relevant data are inside the paper, the Supplementary Textile, and on Figshare (see Supplementary Material two).
Key Words
- action photographic camera
- remote photographic camera
- digital video
- behaviour
- benthic ecology
- science communication
Sections
- Integrative Sciences
Subjects
- Conservation and Sustainability
- Scientific discipline Communication
Authors
Writer Contributions
All conceived and designed the study.
All performed the experiments/nerveless the data.
All analyzed and interpreted the data.
GL and BF contributed resources.
All drafted or revised the manuscript.
Competing Interests
BF is currently serving every bit a Field of study Editor for FACETS, but was not involved in review or editorial decisions regarding this manuscript.
Metrics & Citations
Metrics
Other Metrics
Citations
Cite As
Jonathan A. Bergshoeff, Nicola Zargarpour, George Legge, and Brett Favaro. How to build a low-cost underwater camera housing for aquatic research. FACETS. ii(): 150-159. https://doi.org/x.1139/facets-2016-0048
Export Citations
If you have the appropriate software installed, you lot tin download article commendation data to the citation manager of your choice. Only select your manager software from the list below and click Download.
Cited past
ane. Aqua-Vision (An underwater panoramic photographic camera with computer vision and analytics)
2. Development of a Raspberry Pi-based Underwater Photographic camera System for Inland Freshwater Aquaculture
3. A high-density fish school segmentation framework for biomass statistics in a deep-sea cage
4. FishCam: A low-cost open source autonomous camera for aquatic research
5. A field-based investigation of behavioural interactions betwixt invasive green crab ( Carcinus maenas ), rock crab ( Cancer irroratus ), and American lobster ( Homarus americanus ) in southern Newfoundland
6. Baited remote underwater video estimates of benthic fish and invertebrate multifariousness within the eastern Canadian Chill
7. Improving the efficiency of the Fukui trap equally a capture tool for the invasive European green crab ( Carcinus maenas ) in Newfoundland, Canada
8. Beginning estimates of Greenland shark (Somniosus microcephalus) local abundances in Chill waters
9. Using underwater video to evaluate the performance of the Fukui trap as a mitigation tool for the invasive European green crab ( Carcinus maenas ) in Newfoundland, Canada
View Options
View options
Get Access
Media
Media
Other
Tables
Source: https://www.facetsjournal.com/doi/10.1139/facets-2016-0048
Posted by: jarvisthele1947.blogspot.com

0 Response to "How To Build A Camera Case"
Post a Comment